


But have you ever wondered why certain people smoke and rest don’t? Addiction and susceptibility to smoking and tobacco intake, cessation, and withdrawal symptoms are heritable and are associated with certain genes and genetic polymorphisms. The presence of certain genes leads to an increased risk of developing dependency and addiction towards smoking. This can be understood from the disparity in smoking prevalence and smoking behavior among various races and ethnic groups (Fig. 2). There are genetic differences among populations, races, genders, and even at an individual level. These differences can be attributed to different smoking and cessation behavior.
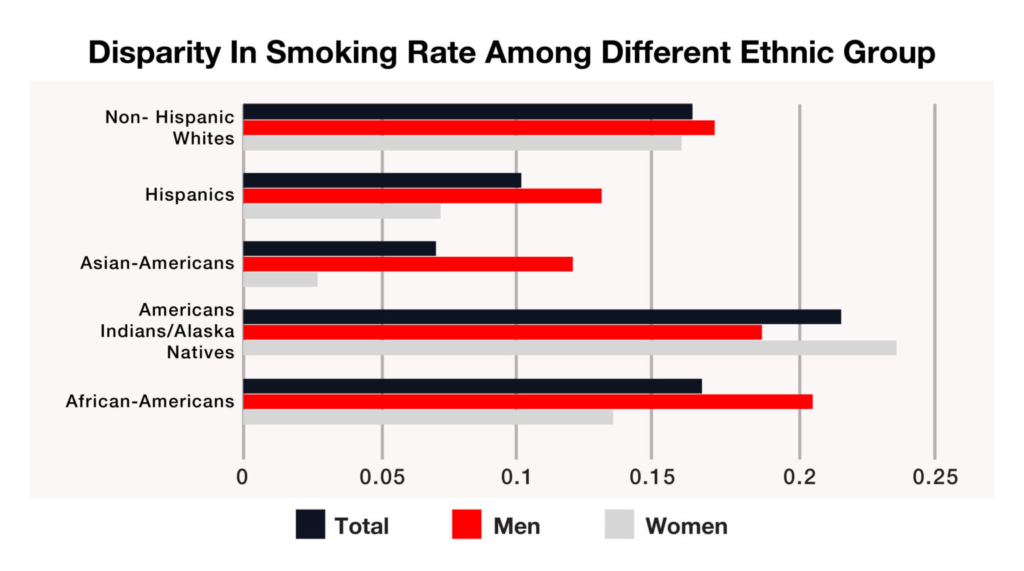
The various environmental factors like demography, socioeconomic factors, and peer pressure associated with smoking and tobacco consumption have been examined previously. But there are some differences among individuals belonging to the same environment. It is rightly said that “Genes load the gun. But lifestyle pulls the trigger.” Genomics implementation of research is increasingly being conducted in behavioral medicine. Cigarette smoking and nicotine use disorder represents prime areas for genomics implementation efforts in behavioral medicine. Individuals without any smoking genes do not feel the urge to initiate smoking or face difficulty quitting. The presence of smoking genes makes a person inclined towards smoking. These individuals adapt the habit of smoking to the slightest environmental instigation.
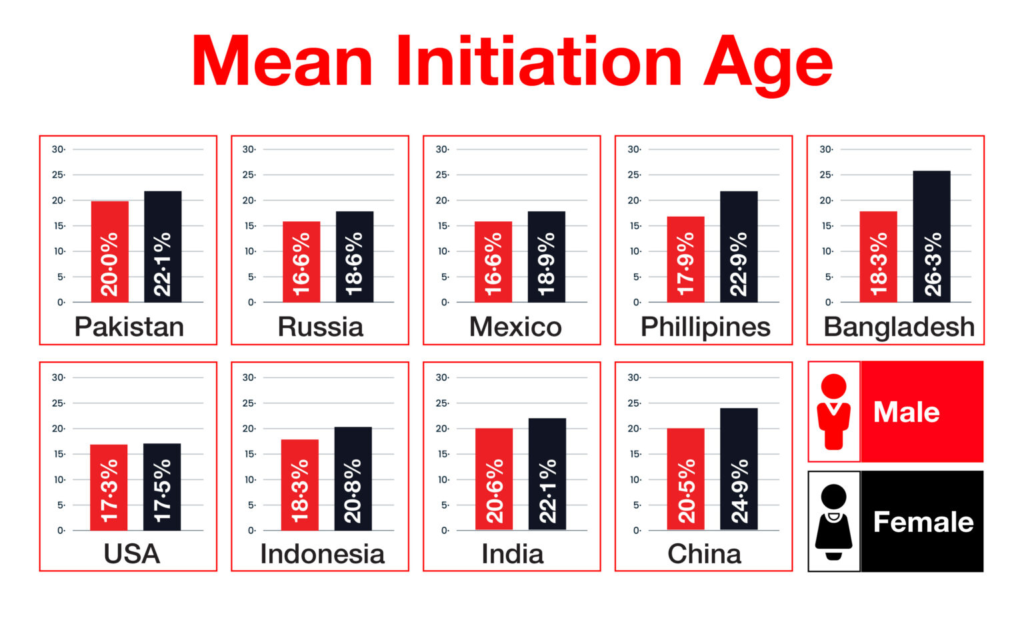
Nicotine is the major component of tobacco that causes addiction. Studies showed that smoking initiation is influenced by both genetic and environmental factors. But, once initiated, it’s the genes that decide the number and quantity of cigarettes and the level of dependency. Age of initiation and smoking behavior are influenced by genes. Studies revealed that several genes cause an individual to become more susceptible to being addicted to nicotine. PITRM1 and DHRS7 for African-American smokers and DDR2 for European-American smokers are new candidate genes for smoking initiation and are being studied thoroughly. These genes represent genetic influences on smoking initiation, an important but less studied phenotype in nicotine dependence research. A study conducted by the Institute for Health Metrics and Evaluation in 2021 revealed that the age of initiation of smoking differs among various populations (Fig. 3).
Nicotine dependence is associated with a single nucleotide polymorphism in the DMT3 gene. The polymorphism can occur in the fetal stage or in the adult stage. This polymorphism is found in both heavy and mild smokers. The gene is also associated with smoking-induced lung cancer.
The number of cigarettes smoked per day varies for every individual. It has been found that smokers carrying a homozygous CHRNA mutated gene, smoke significantly more tobacco than non-carriers.
The pattern of smoking and smoking behavior is drastically different among individuals. African-American smokers, on average, consume fewer cigarettes daily, inhale more deeply, and break down nicotine more slowly than European-American smokers. This is due to the biochemical differences between the two groups. Genome-wide analyses conducted from 2006 to 2018 showed that the genetic differences on chromosomes 4, 10, 13, and 19 between the two groups are the reason for the disparity in smoking patterns and nicotine metabolism.
Various psychological disorders, like depression, are found to be genetically associated with smoking. It has also been established that there is a genetic association between smoking and BMI. The genetic predisposition for the consumption of cigarettes appears to be linked to the genetic predisposition for a higher BMI, or obesity. It is known that a habitual or occasional smoker craves smoking in response to certain environmental stimuli, such as stress. Individuals carrying DRD2 (D2 dopamine receptor gene) A1 or the SLC6A3 (dopamine transporter gene) genes have been reported to experience a significantly stronger stress-induced craving. A person harboring both of these genes has an irresistible craving for smoking when stressed.
Gender also plays a major role in the differences in smoking behavior. Smoking activates men’s reward pathways more than women’s. These are due to genetic differences between male and female nicotine receptor genes. The choice of tobacco products and cigarette types is gender biased. These differences in choices are due to gender-specific psychological, hormonal, and behavioral genes.
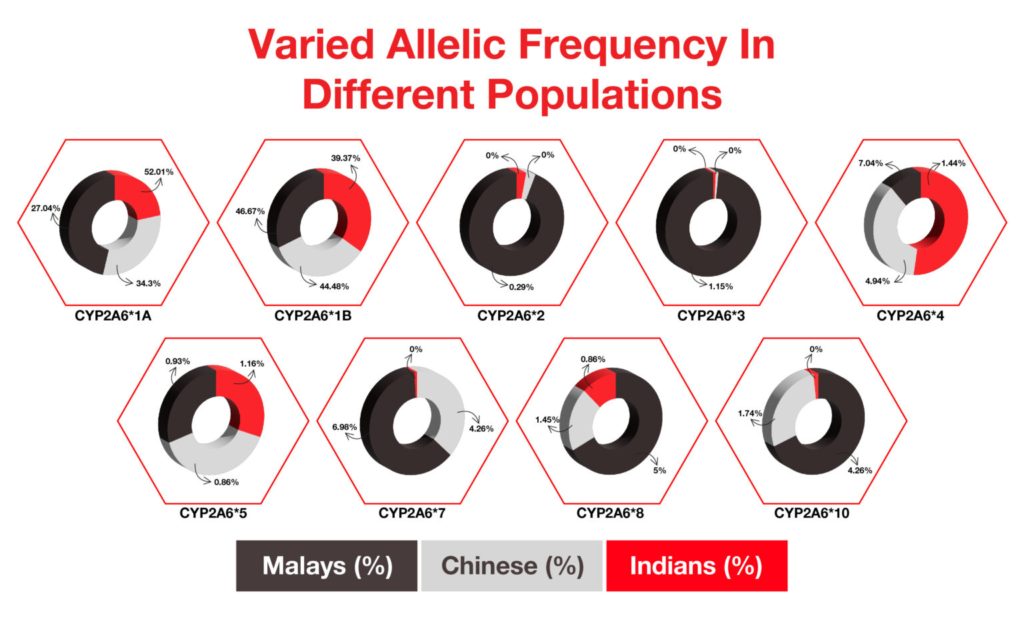
The pharmacokinetics of nicotine have already been studied elaborately. In the body, nicotine is metabolized to cotinine and trans-3-hydroxycotinine by the enzyme liver cytochrome P450 (CYP). This enzyme is encoded by the gene CYP2A6. Variation in this gene can lead to altered smoking habits. Polymorphisms in CYP2A6 were found in Alaska Native and American Indian (AN/AI) populations but absent in other population groups. This is one of the reasons for the high prevalence of smoking and tobacco in this group. Variations in CYP genes were thoroughly studied in 2006 in Indian, Chinese, and Malay populations residing in Malaysia. A significant difference has been found in the alleles of these populations (Fig. 4).
After inhaling tobacco smoke, nicotine is transported to the brain in ten seconds through the lungs and bloodstream. In the brain, nicotine binds to ligand-gated nicotinic cholinergic receptors (nAChRs), which usually bind to acetylcholine. This results in the opening of the ion-channel, which allows the entry of Na+ and Ca2+ cations. This, in turn, leads to the activation of voltage-dependent calcium channels, causing an influx of calcium to nerve cells and, eventually, the release of neurotransmitters. The α4 subunit is a determinant of sensitivity to nicotine. It often exists together with a β2 subunit, which has the strongest nicotine affinity, and some other adjusting subunits. The CHRNA5-CHRNA3-CHRNB4 gene cluster encoding three nicotinic acetylcholine receptor subunits is associated with smoking behaviour. Polymorphisms in these gene clusters result in lower Ca2+ permeability, which reduces nicotine signaling and could thus lead to heavier smoking.
Another gene, SLC6A3, influencing the dopamine pathway, is associated with the feel good syndrome of smoking. Mutation or variation in this gene can increase or decrease the desire for smoking based on the type of mutation. A review analysis conducted in 2004 showed that the common variants of this gene are a ten-repeat allele found in 70% of European, Caucasian, or African-Americans and 90% of Asian or South American populations. The nine-repeat allele is found in 25% of European, Caucasian, or African-Americans.
The dopamine receptor gene (DRD) is also associated with smoking behavior. Polymorphisms in DRD genes lead to decreased dopamine receptor expression. Individuals with this polymorphism tend to smoke more to compensate for the reduced dopamine levels.
Monoamine oxidases (MAOs) catalyze the deamination of amines such as dopamine, noradrenaline, and serotonin. This gene is also involved in smoking behavior. Mutations in this gene leading to reduced expressions of the neurotransmitters can cause a person to become a heavy smoker.
The neurogenes are responsible for the cessation and quitting behaviors of individuals. Racial or ethnic differences are also observed in successful quitting and smoking relapse. In a particular US-based study carried out in 2017, it was observed that Non-Hispanic White adults reported more withdrawal symptoms, withdrawal-related discomfort, and withdrawal-related distress than non-Hispanic Black and Hispanic adults. Certain polymorphisms cause resistance to smoking cessation and no desire to stop smoking. Craving to smoke is a major challenge for those who try to quit smoking. Single nucleotide polymorphisms in Galanin (GAL) and its receptors (GALRs) are associated with the severity of craving.
Stress also plays a role in cessation and withdrawal symptoms. Smokers prone to stress have severe withdrawal symptoms and a greater risk of smoking relapse. The FKBP5 gene is associated with stress, and variations in this gene can affect the severity of withdrawal symptoms and the chances of relapse.
The risk of relapse always persists, especially in heavy smokers. It is known to be triggered by environmental stimuli. A genome association study carried out in 2018 showed that a polymorphism in the CHRNA5 gene is associated with the risk of nicotine relapse. The mutation is highly present in approximately 35% of Europeans and up to 50% of the population of the Middle East. The relapse is associated with reduced neuronal activation in the interpeduncular nucleus, a brain structure with the highest concentration of α5 nicotinic receptor subunits. Former smokers with increased nicotine receptors are at a higher risk of relapse.
FDA-approved pharmacotherapies and behavioral therapies are used to treat smoking addiction. Medications like varenicline, bupropion, and anti-depressants are generally used. However, individuals with multi-drug resistant genes or specific neurogenic polymorphisms are not affected by these drugs. The urge to smoke is not reduced by such treatments.
To effectively help a person to let go of smoking behavior, the genotype of the individual must be studied. Smoking must be treated as a genetic disorder and treated accordingly. Varenicline is expected to be more effective in smokers with genotypes associated with reduced dopamine availability. Since nortriptyline has antidepressant properties, like bupropion, nortriptyline is expected to be more effective in smokers with genotypes associated with increased dopamine availability. The genetic influence on withdrawal symptoms are chances for relapse must be analyzed and later planned for therapy. Likewise, nasal sprays are more beneficial compared to transdermal patches for individuals with reduced expression of nicotine or dopaminergic receptors because of the more rewarding effects of nasal sprays. Individuals with increased expression of the μ-opioid receptor (MOR) benefit better from transdermal nicotine patches. It has been found that nicotine replacement therapies do not influence individuals with altered serotonin pathways.
These show that a single therapy cannot be beneficial for all. While treating smoking nicotine addiction, or any addiction at large, each individual is different and should have a specific, personalized treatment plan. There has been very limited research on the genetics of the various Indian populations associated with smoking. But global studies show that smoking patterns are highly influenced by genes. To acquire a tobacco free country, the focus must be transferred deeper to the genetic predisposition of the individuals. The existence of an individual is based on his on her genes. The various combinations of the four nucleotides determine appearance, body functioning, behavior, way of thinking, susceptibility to infections, diseases, and addiction. With the advancement of technology, the human genotype can be easily analyzed. If the genotypes are known, the treatments can be successfully used to eradicate addictions.
Bozkurt, Nurgul et al. Effect of MDR C3435T polymorphism on Varenicline treatment in quit smoking. Brazilian Journal of Pharmaceutical Sciences [online]. 2019, v. 55
Chenoweth MJ, Ware JJ, Zhu AZX, et al. Genome-wide association study of a nicotine metabolism biomarker in African American smokers: impact of chromosome 19 genetic influences. Addiction. 2018;113(3):509-523. https://doi.org/10.1111/add.14032
Erblich, J., Lerman, C., Self, D. et al. Stress-induced cigarette craving: effects of the DRD2 TaqI RFLP and SLC6A3 VNTR polymorphisms. Pharmacogenomics J 4, 102–109 (2004). https://doi.org/10.1038/sj.tpj.6500227
Forget B et al. A Human Polymorphism in CHRNA5 Is Linked to Relapse to Nicotine Seeking in Transgenic Rats. CB. https://pubmed.ncbi.nlm.nih.gov/30293722/. Accessed July 14, 2021.
GATS2 (Global Adult Tobacco Survey) Fact Sheet, India, 2016-17. https://www.who.int/tobacco/surveillance/survey/gats/GATS_India_2016-17_FactSheet.pdf. Accessed July 14, 2021.
Global Adult Tobacco Survey Fact Sheet.pdf. http://www.aftcindia.org/pdf/File%2010.pdf. Accessed July 14, 2021.
Jiang K, Yang Z, Cui W, et al. An Exome-Wide Association Study Identifies New Susceptibility Loci for Age of Smoking Initiation in African- and European-American Populations. Nicotine Tob Res. 2019;21(6):707-713. https://doi.org/10.1093/ntr/ntx262
Lori, A., Tang, Y., O’Malley, S. et al. The Galanin Receptor 1 Gene Associates with Tobacco Craving in Smokers Seeking Cessation Treatment. Neuropsychopharmacol 36, 1412–1420 (2011). https://doi.org/10.1038/npp.2011.25
Maserejian NN, Zavras AI. Genetics of tobacco use. Tob Induc Dis. 2004;2(2):81-102. Published 2004 Jun 15. https://doi.org/10.1186/1617-9625-2-2-81
M. Nurfadhlina, K. Foong, L. K. Teh, S. C. Tan, S. Mohd Zaki & R. Ismail (2006) CYP2A6 polymorphisms in Malays, Chinese and Indians, Xenobiotica, 36:8, 684-692, https://doi.org/10.1080/00498250600715932
National Institute on Drug Abuse. Are there gender differences in tobacco smoking? National Institute on Drug Abuse. https://nida.nih.gov/publications/research-reports/tobacco-nicotine-e-cigarettes/are-there-gender-differences-in-tobacco-smoking. Published April 12, 2021. Accessed July 14, 2021.
NIDA. “Are there gender differences in tobacco smoking?.” National Institute on Drug Abuse, Accessed on 12 Apr. 2021,
Reitsma, M., Flor, L., Mullany, E., Gupta, V., Hay, S. and Gakidou, E., 2021. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990–2019.
The Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 42, 441–447 (2010). https://doi.org/10.1038/ng.571
Wills AG, Hopfer C. Phenotypic and genetic relationship between BMI and cigarette smoking in a sample of UK adults. Addict Behav. 2019;89:98-103.